Steam Tables: Vapor, Liquid and Solid Relations
Steam Tables: Vapor, Liquid and Solid Relations
2000 - 2001
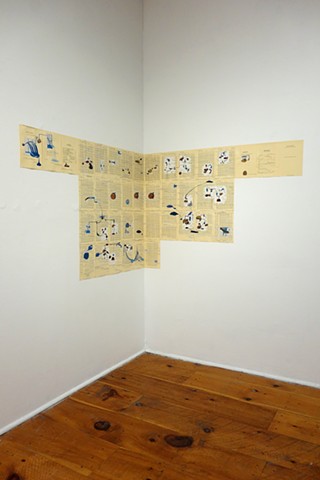
Installation
Mixed media on book pages (42 pages)
10" x 6 ½” each
Installation size: 14’ x 2 ½’
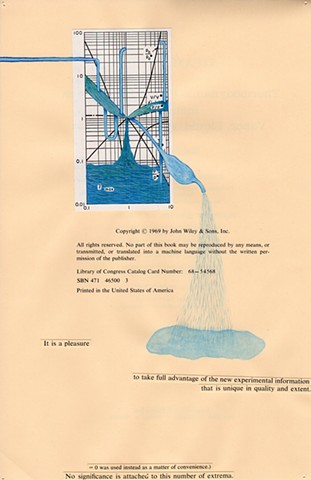
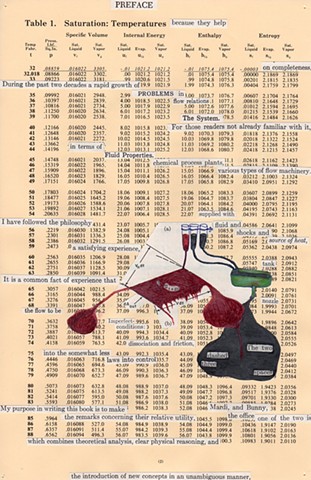
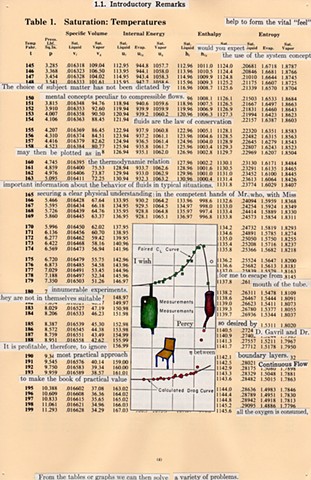
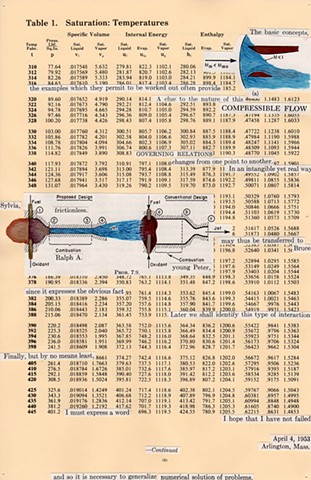
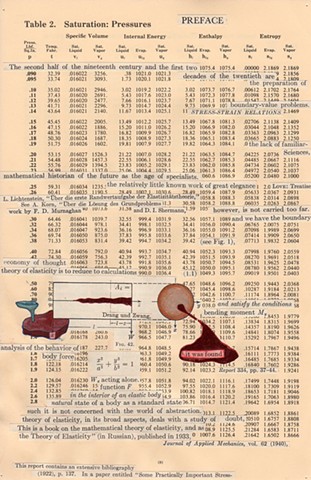
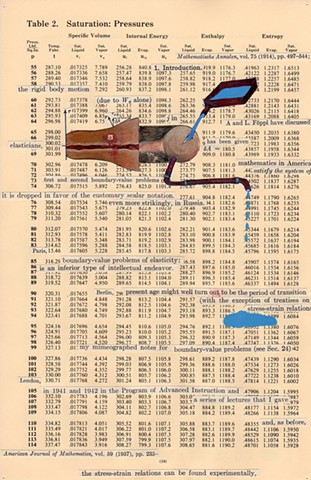
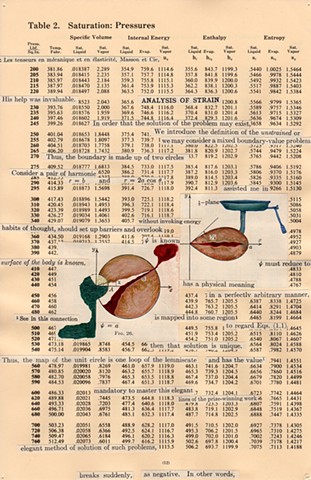
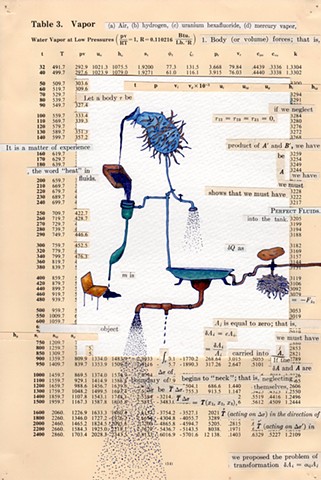
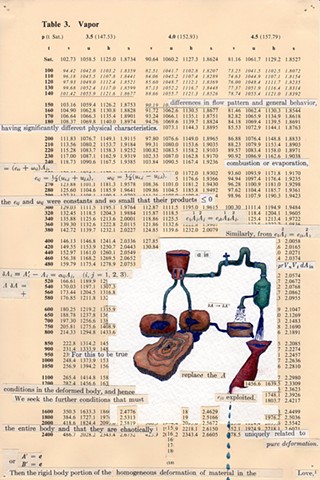
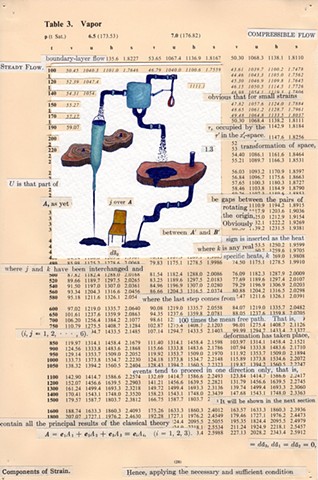
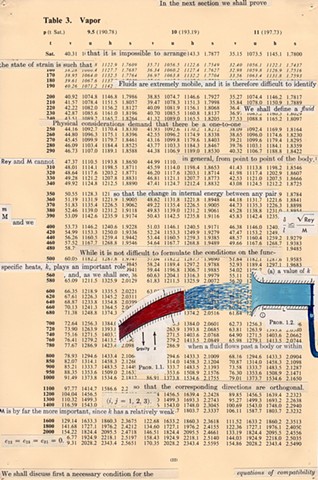
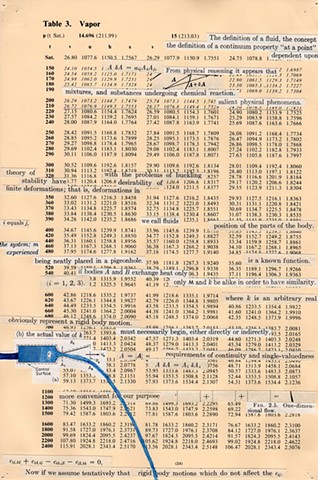
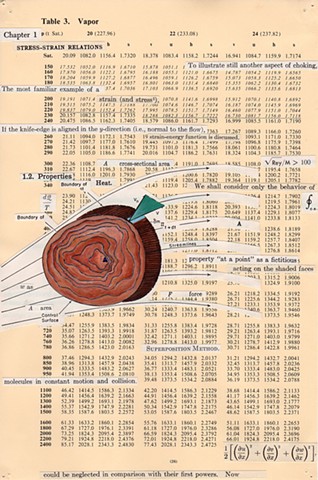
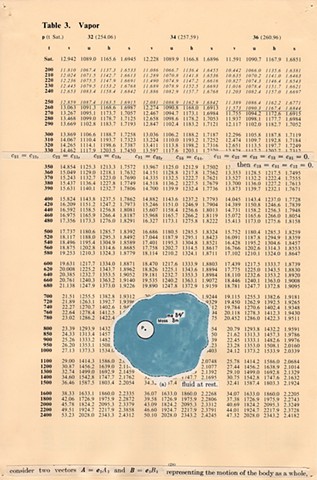
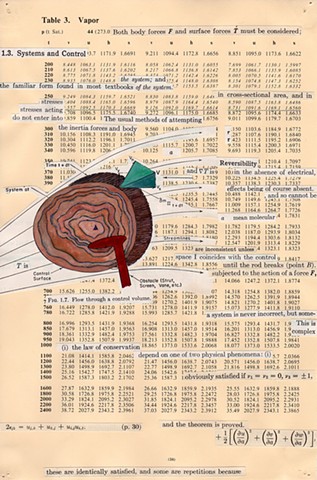
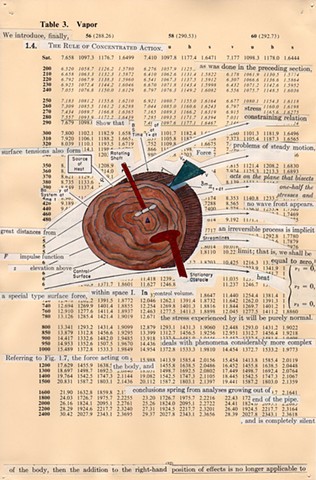
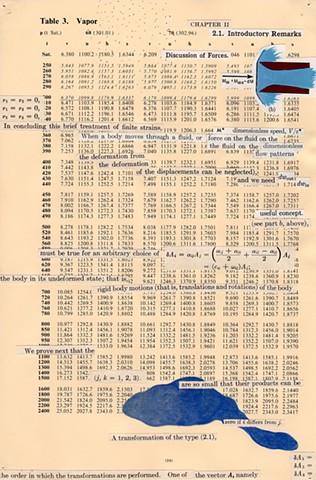
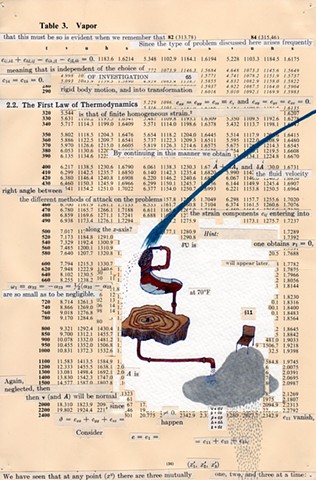
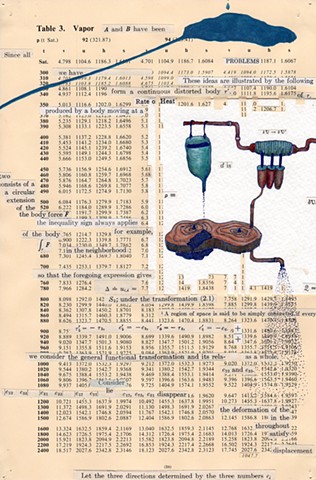
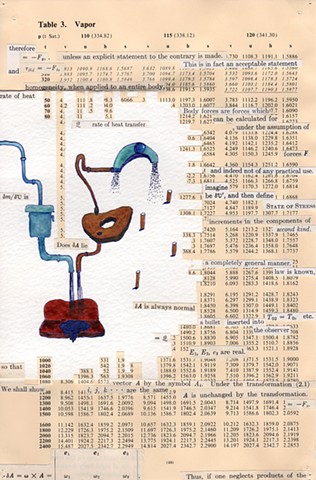
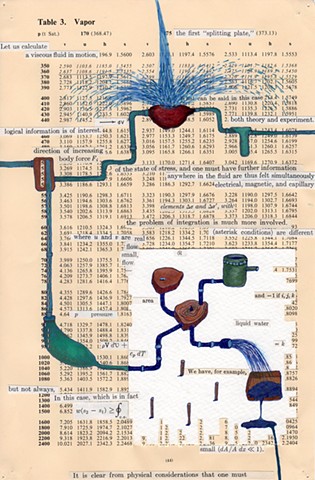
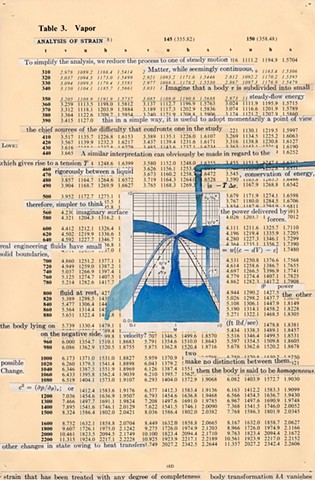
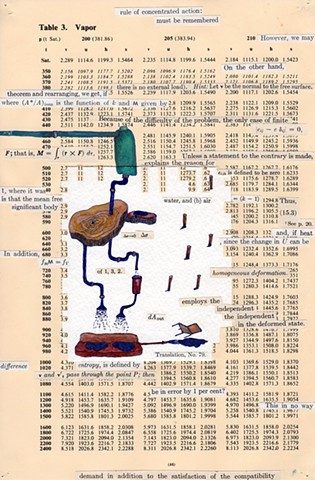
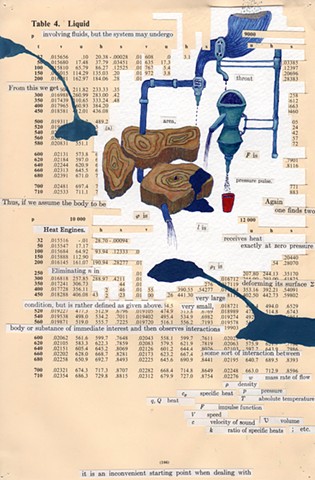
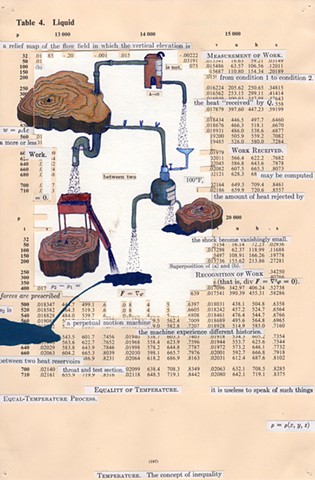
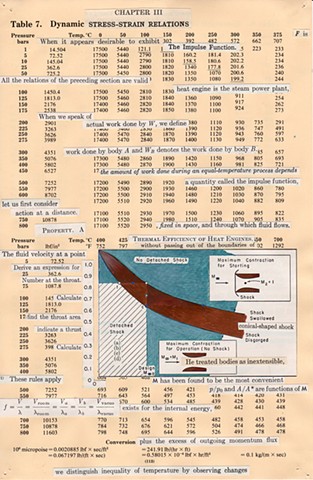
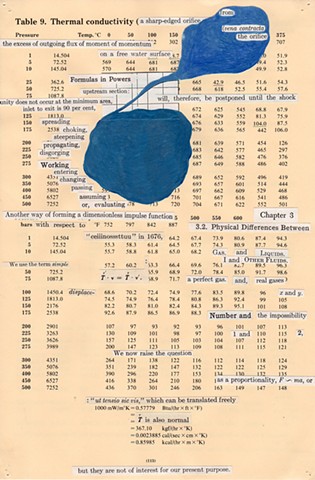
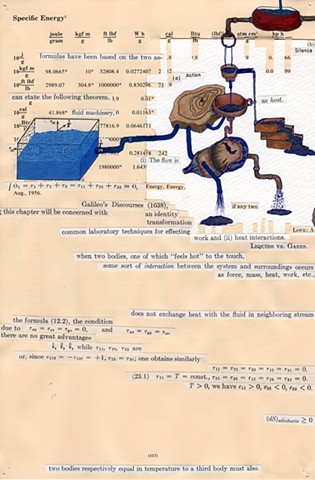
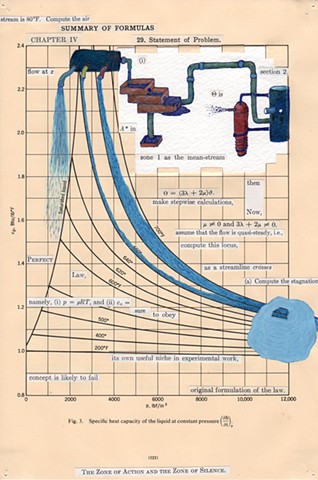
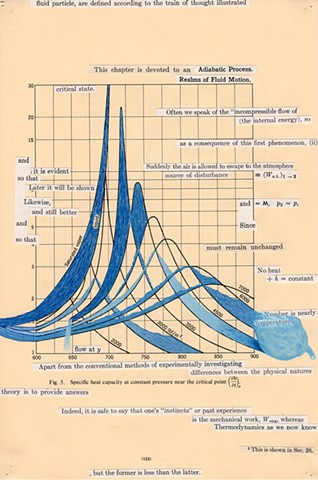
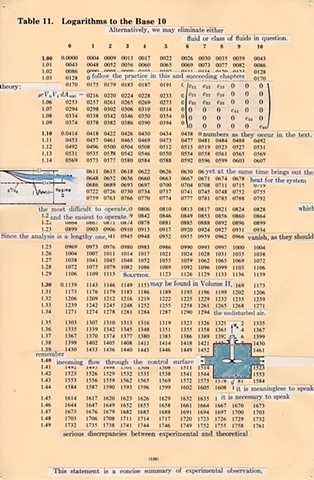
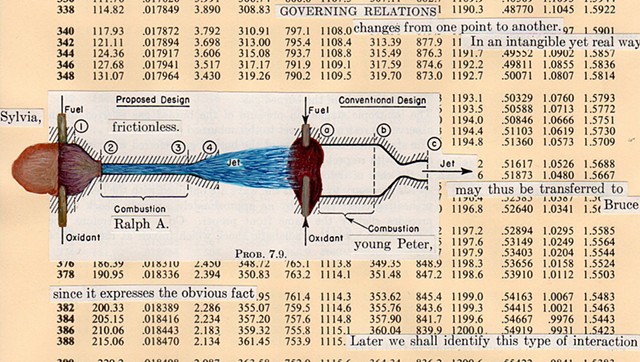
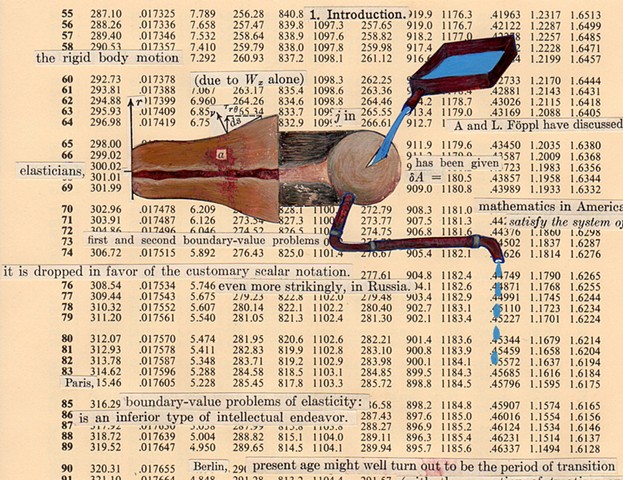
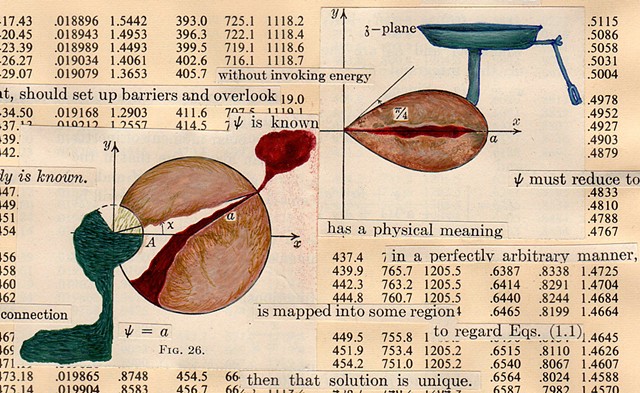
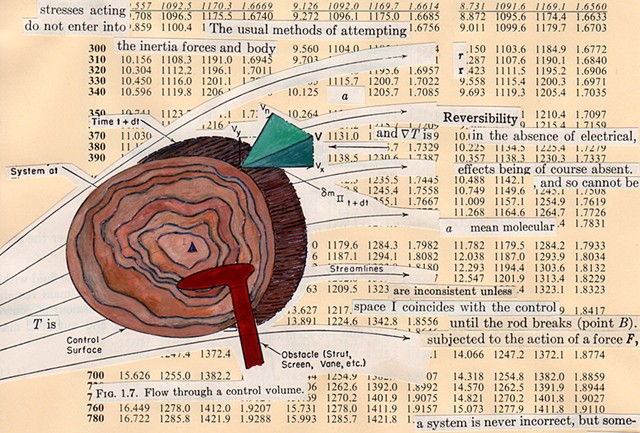
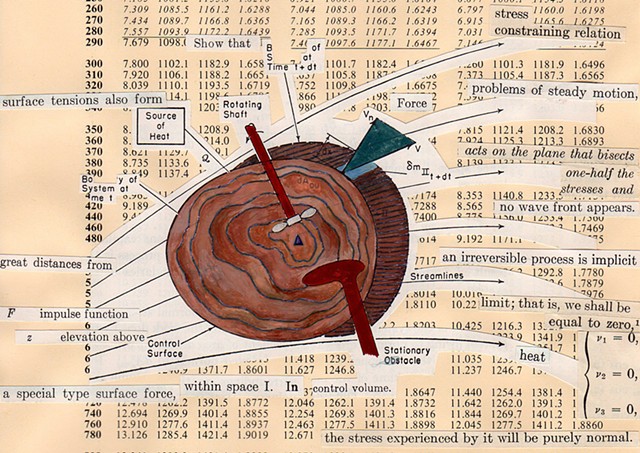
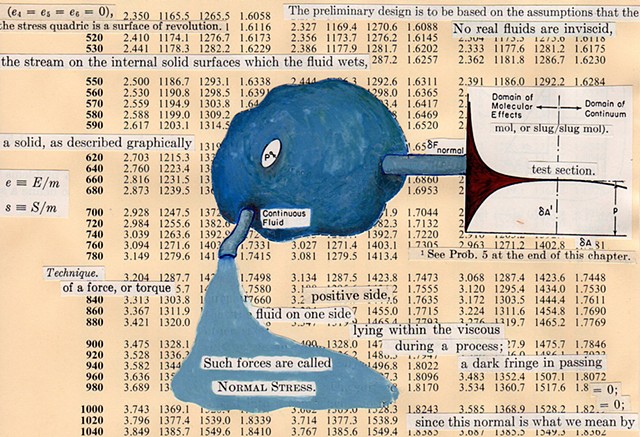
I used physics and mathematical information to develop a theory on relationships. Following the order of a book from the face page, content, preface, chapter I, II and III and appendix and so on, I created different levels of narratives on these pages. Texts on the bottom of each page can be read from page to page separately, while the images of fluids spill over and connect pages to create another level of narratives. Texts and altered images from two books, The Dynamics and Thermodynamics of Compressible Fluid Flow (1953) and Mathematical Theory of Elasticity (1946), are combined onto pages from Steam Tables: Thermodynamic Properties of water Including Vapor, Liquid and Solid Phases (1969), in which the book was introduced as having the first time high-quality experimental data on liquid and vapor water that are represented by a single fundamental equation. From this equation all thermodynamic properties can be calculated for any state. Using this data as background, I layered more information on each page, such as equations, illustrations or texts from two other sources and made drawings, creating visual experiments and equations for relationships—dynamic and fluid as water is from a state of being solid, liquid, to vapor.
a relief map of the flow field in which the vertical elevation is
Measurement of work
from condition 1 to condition 2
the heat “received” by Q,
w=pAc
a more or less
Work.
WORK RECEIVED.
between two
100°F, may be computed
the amount of heat rejected by
=0.
the shock becomes vanishingly small.
Superposition (a) and (b),
RECOGNITION OF WORK
P2-P1= (that is, div F=).
F=
forces are prescribed
P2 is
a perpetual motion machine
The machine experiences different histories.
between two heat reservoirs
throat and test section.
EQUALITY OF TEMPERATURE
EQUAL-TEMPERATURE PROCESS. it is useless to speak of such things
p=p(x,y, z)
(107)
TEMPERATURE. The concept of inequality
References
1. Keenan, J. H. , Keyes, F.G., Hill, P.G. and Moore, J.G. Steam Tables: Thermodynamic Properties of water Including Vapor, Liquid and Solid Phases: English Units. New York: John Wiley & Sons, Inc., 1969.
2. Shapiro, A.H. The Dynamics and thermodynamics of Compressible Fluid Flow. New York: John Wiley & Sons, Inc., 1953.
3. Sokolnikoff, I.S. and Specht, R.D. %Mathematical Theory of Elasticity.& New York: McGraw-Hill Book Company, Inc., 1946.